New design strategies towards more effective catalysts*
|An efficient catalytic process is crucial for several applications, such as energy production in fuel cells and catalytic converters in cars. Precious noble-metals such as platinum play a key role in the catalysis and their use must be optimized to reduce costs and maximize their potential. Single-atom catalysts maximize the utilization of supported precious metals by exposing every single metal atom to reactants. Although this concept is widely known, preserving the stability of single atoms on a support material under working conditions (e.g. high temperature) is still a major challenge.
Filip Dvořák and Matteo Farnesi Camellone, with their colleagues from the Charles University in Prague, CNR-IOM DEMOCRITOS in Trieste and SISSA in Trieste, combined CERIC highly sensitive photoelectron spectroscopy with scanning tunneling microscopy and density functional theory calculations, to explore the physics and chemistry behind the exceptional activity of ceria-based catalysts with an atomic dispersion of ionic platinum.
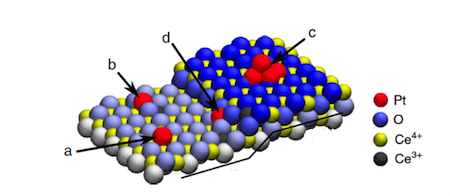
Dvorak’s and Camellone’s study shows that monoatomic step-edges, which are the most pervasive defects on solid surfaces such as the support material ceria, provide specific structural and electronic environments for the selective formation of uniform, thermally and chemically stable Pt2+ ions. Moreover, they found that the platinum ions are stabilized as platinum oxide (PtO4), which can provide additional reactivity in oxidation reactions.
Experimentally controlling the engineering and decoration of the steps, as in the present study, may bring about a more effective use of precious metals in the catalytic processes, for less expensive and more environmentally friendly energy production.



