Novel approach to production of cost efficient platinum-based catalysts
|The little things are infinitely the most important. — Arthur Conan Doyle.
We are speaking of catalysts, tiny particles of noble metals invisible to the human eye. Wherever these are employed, in the exhaust system of cars or in chemical plants, their purpose is to lower the energy consumption needed for a chemical reaction to take place, or to let it run more efficiently, without being consumed. This is why they have become more important as the world turns to more efficient and climate friendly uses of energy. In the last years, great progress has been made in the development of tailor-made catalysts for various purposes, including production of synthetic fuels and energy conversion in batteries and fuel cells. Typically, catalysts consist of a noble metal, like platinum, placed on oxide materials. The high price of noble metals drives the development of novel catalysts with the lowest amount of precious metals.
One possible solution involves the use of highly dispersed sub-nanometer-sized platinum particles. These particles have the highest surface to volume ratio, which makes them the most efficient materials in terms of active area per total noble metal loading. However, the stabilization of such particles against sintering during catalyst operation is challenging. In order to increase the stability of these small particles, the team of Yaroslava Lykhach and Joerg Libuda from the University of Erlangen Nuremberg in Germany, developed a new strategy for catalyst preparation. This strategy makes use of charge transfer phenomena in solids, known as redox interactions. Previous studies have shown that platinum can be atomically dispersed in the form of positively charged platinum ions anchored on a cerium oxide support. In this material, the formation of highly stable sub-nanometer platinum particles can be triggered by charge transfer from the cerium oxide to the platinum ions in the presence of oxygen vacancies. It is also known that tin is an effective reducing agent for cerium oxide, inducing charge transfer to the platinum ions.
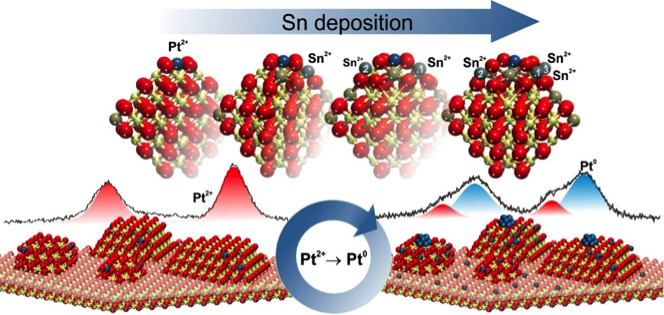
Lykhach’s team used these two facts to produce sub-nanometer platinum particles without the need to create oxygen vacancies. The corresponding experiment was performed at the Materials Science beamline at the CERIC Czech Partner Facility in Trieste. They found out that certain concentrations of tin result in the complete conversion of platinum ions to catalytically active platinum particles. This process was observed by means of Synchrotron Radiation Photoelectron Spectroscopy (SRPES) and Resonant Photoemission Spectroscopy (RPES). The depth distribution of tin in the cerium oxide was monitored by Angled Resolved X-ray Photoelectron Spectroscopy (ARXPS) at Charles University in Prague. Although the catalytic performance still needs to be tested, the results are already an important step towards production of cost efficient catalysts.



