Breakthrough towards the understanding of electrochemical processes in modern electrocatalysts
|The global transition to a clean, renewable energy system stimulates the development of new technologies for the conversion of chemical energy to electricity. Many emerging technologies including, fuels cells, water splitting, and energy storage devices, are based on principles of electrocatalysis. Essentially, electrocatalysis is a catalytic process which occurs at the surface of a solid electrode immersed into a liquid electrolyte. The electrolyte is an electrically conducting aqueous solution in which chemical reactants are added. The chemical reaction is driven by applying a potential to the electrode. In order to increase the reaction speed of an electrochemical process, the surface of the electrode is plated with an electrocatalyst.
To date, the most active and efficient electrocatalysts contain nanoparticles of noble metals on supports. The activity of such electrocatalysts critically depends on the structure of the adsorption sites. The rational design of active electrocatalyst requires the identification of the most active sites. However, large variety of adsorption sites in real electrocatalytic materials makes it difficult to establish a link between the activity and the structure of the adsorption site. A general approach to overcome this problem is to use structurally well-defined model systems of real electrocatalytic materials.
Model systems of different structural and chemical complexity can be prepared in ultra-high vacuum (UHV) ranging from simple surfaces such as metal single crystals to complex ones using supported metal nanoparticles. However, the transfer of model systems from the UHV to a liquid electrochemical environment represents a great challenge. Specifically, such a transfer must be carried out under conditions that preserve the well-defined structure and cleanliness of the model systems. For example, exposure of the model surfaces to air results in contamination and deactivation of catalytically active sites. In the field of electrochemical surface science, the catalyst surface is often cleaned by means of a flame annealing. While this cleaning procedure can be used with single metal crystals such as platinum, e.g., Pt(111), it causes irreversible damage to more complex systems such as supported metal nanoparticles.
The research team around Prof. Dr. Jörg Libuda from the University of Erlangen-Nuremberg in Germany made a breakthrough in this direction. In an article published in the prestigious journal Nature Materials, they describe a new strategy by which it is possible to study a complex model catalyst in the form of Pt nanoparticles supported on well-ordered cobalt oxide Co3O4(111) films in a liquid environment. To this end, they used a special setup that allows the transfer of the model catalyst from UHV into a liquid electrolyte, without exposing its surface to air. In the study, numerous techniques were applied including Synchrotron Radiation Photoelectron Spectroscopy (SR-XPS) at the Material Science Beamline and X-ray Photoelectron Spectroscopy (XPS) coupled with an electrochemical cell at the CERIC Czech Partner Facility in Trieste and Prague, respectively.
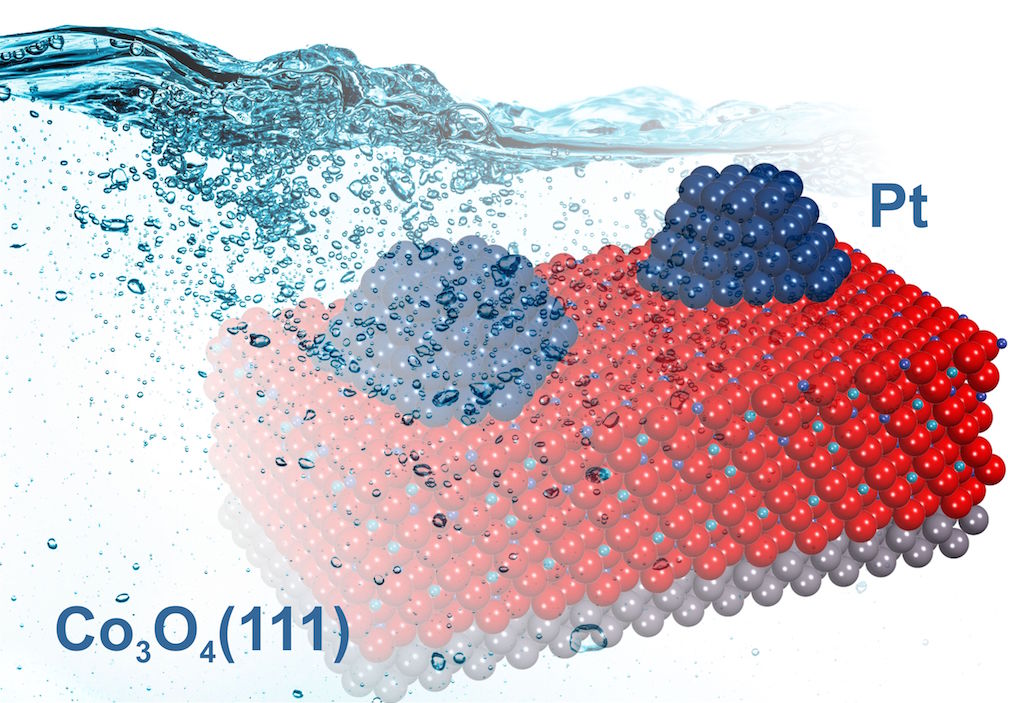
Introducing well-defined model electrocatalysts into the field of electrochemistry
The researchers proved the potential of the concept, which makes available a systematic approach to build complex, yet atomically defined model electrocatalysts for fundamental electrocatalytic studies. This novel strategy might pave the way towards the development of more effective, stable and cost-efficient catalysts to be applied, among others, in systems producing clean energy.



