CERIC scientists look into details the degradation of bimetallic alloy catalysts
|A Fuel Cell is a device that converts the chemical energy of a fuel into electricity without burning it. Together with batteries, they represent a relevant alternative for the future of energy and mobility. Among the many technologies developed over the years, Proton Exchange Membrane Fuel Cells (PEMFC) gathered much attention for their interesting features, such as high power density and low operational temperatures.
The catalyst is a fundamental component of PEMFC; however, being mostly made of platinum, it also represents a challenge. Nowadays, many efforts are put towards the substitution of platinum, or the minimisation of its employment. The latest work of the CERIC’s internal research project CEROP follows this purpose.
The work, led by Dr. Ivan Khalakhan (Charles University of Prague) and Professor Heinz Amenitsch (Graz University of Technology), in collaboration with Marco Bogar (CERIC-ERIC), Serhiy Cherevko (Forschungszentrum Jülich GmbH) and colleagues, highlighted the behavior of a fuel cell catalyst made of an alloy of platinum and nickel (PtNi). In this case, the costs are lower, but durability at operational conditions becomes an essential factor.
The study revealed the correlation between the upper potential limit during potentiodynamic cycling, which represents a different fuel cell operation regime, and the structural behavior of the PtNi catalyst by employing state-of-the-art in situ techniques. Among them, grazing-incidence small-angle X-ray scattering (GISAXS) and Atomic Force Microscopy (AFM), available respectively at the Austrian and Czech Republic CERIC partner facilities. AFM was used to explore microscopic processes involved in the degradation of the catalyst, while GISAXS investigated the structural changes at the interface (parallel and perpendicular to the surface) between 1 and 100 nanometers.
The study showed that at lower upper potentials (0,6 and 1 V) the catalyst keeps its morphology during the entire cycling procedure. In contrast, dramatic changes occur in the structure at higher upper potentials (1,3 and 1,5 V). In the latter condition, nickel dissolution took place rapidly at early stages of cycling, which is substituted by catalyst coarsening at later stages. The latter phenomenon identifies the growth of the mean particle size driven by the reduction in particle boundary area.
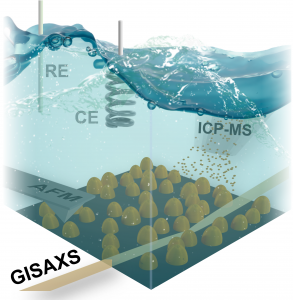
The use of well-defined conditions and sensitive in situ experiments revealed detailed information about the degradation of bimetallic alloy catalysts, providing valuable insights. The GISAXS technique will provide new possibilities for experimental investigations not only for fuel cell technology, but also for batteries, supercapacitors or electrochemical processes on electrode surfaces in general, in which detailed structural information is essential.
CEROP research project was featured on the science radio program RADAR, produced by the Italian broadcasting service RAI (in Italian). Below you can find the interview with Marco Bogar and Simone Pollastri.



