Oxidised nanosheets with improved electrocatalytic performances
|The global demand for energy and environmental sustainability represent critical challenges of this century. Photocatalysis and electrocatalysis are key processes to drive and accelerate the production of fuels, to reduce gas emission and produce renewable energy sources. Water splitting is a crucial reaction with remarkable energy applications. Water can, in fact, be split in its constituents, oxygen and hydrogen, through fundamental photocatalytic and electrocatalytic reactions, such as hydrogen evolution (HER) and oxygen evolution (OER) reactions. Both academia and industry showed a great interest in the possibility to develop and study new materials to boost overall energy production and enable effective energy storage, as green hydrogen through photocatalysis and electrochemistry.
In a research work, coordinated by Professor Antonio Politano from the University of L’Aquila, the oxidation of nanosheets of gallium selenide (GaSe) and indium selenide (InSe) was tested by both experiments and theory, with relevant innovative features. In fact, their exfoliation in atomically thin layers enhances their performances in electrochemistry and photocatalysis. Experiments showed that the increase in performance is not originated by an increase in the surface-to-volume ratio, but rather to the formation of a thin oxide skin, which represent the catalytically active interface.
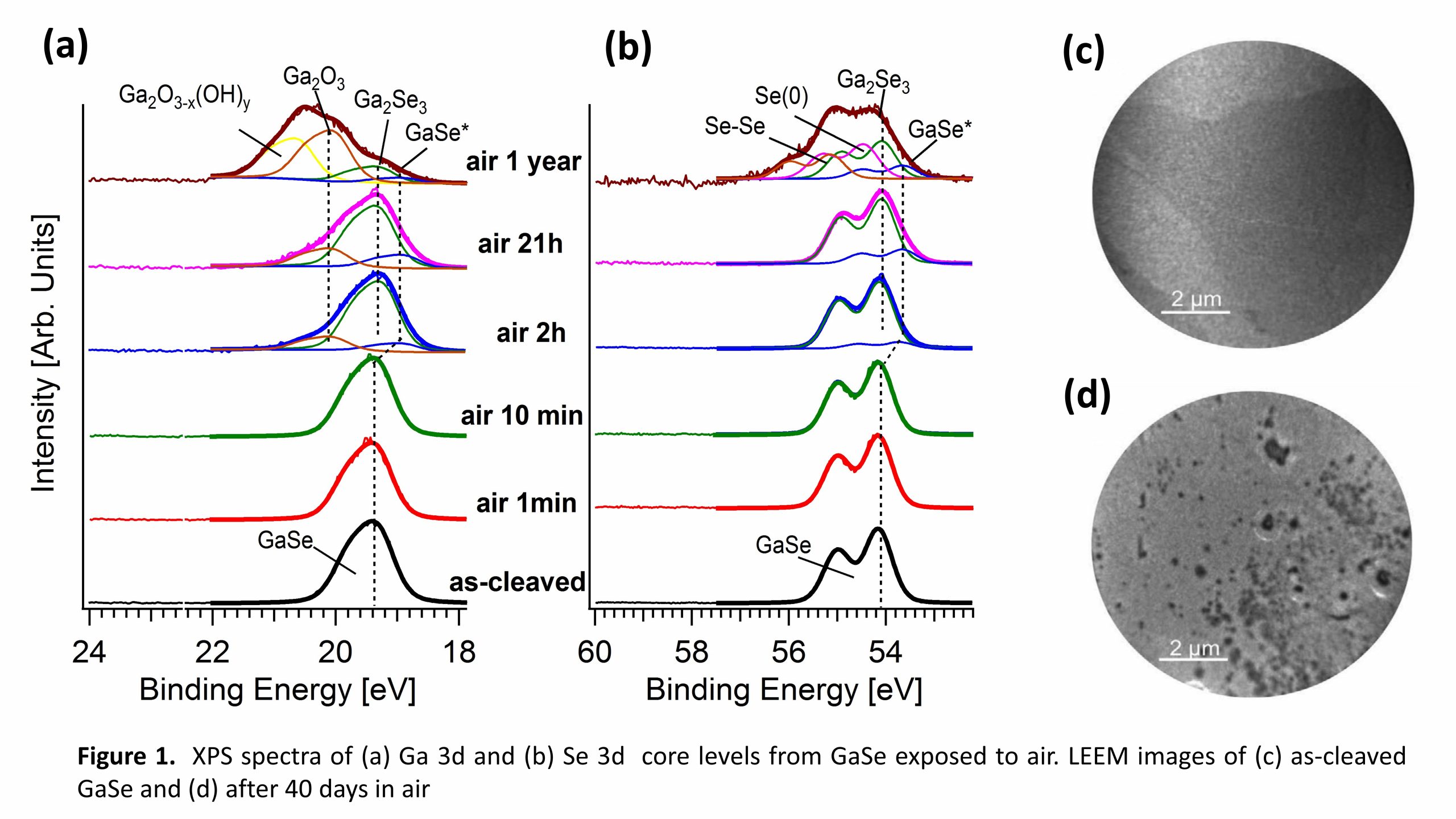
Among the experimental techniques employed in this research work, there’s the Low-Energy Electron Microscopy (LEEM), available at the Nanospectroscopy beamline of the Italian CERIC partner facility in Trieste, the Elettra synchrotron.
These findings pave the way for a novel generation of efficient and cost-effective (photo-)electrocatalysis.



