Na-ion batteries: understanding molecular structure to explain performance
|Metal hexacyanoferrates, such as the ones realized with manganese (MnHCF), are promising positive electrode materials for organic rechargeable batteries, including Na-ion ones, because of their large specific capacity, high discharge potential and sustainability.
When developing these new devices, however, it is important to understand in detail the influence that various factors can have on the molecular structure of the used materials, and thus on performance.
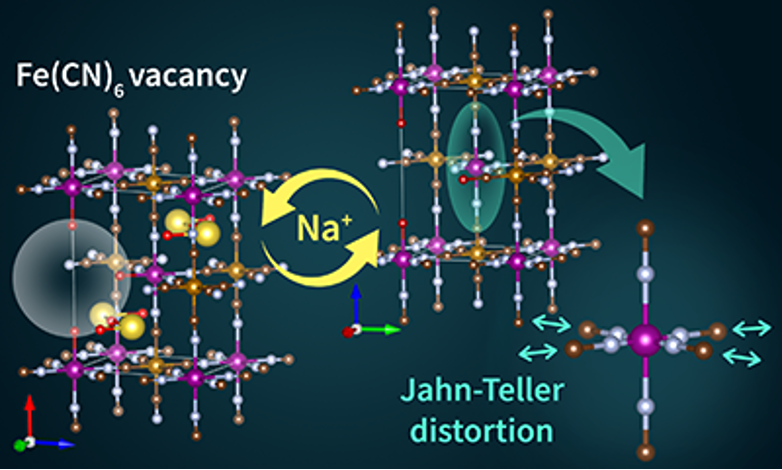
Dr Min Li, Dr Mariam Maisuradze, Prof. Marco Giorgetti and colleagues of the University of Bologna synthesized two MnHCF materials with the same phase, similar particle size, but different [Fe(CN)6]4− vacancy content (respectively 4 % and 11 %), and tested them as cathode material in organic Na-ion battery.
They first demonstrated that the material with the lower vacancies exhibits higher capacity retention (71.1 % vs. 39.4 %) after 100 cycles. Moreover, applying X-ray absorption spectroscopy (XAS) and ex situ X-ray diffraction (XRD) testing, both available at the CERIC Italian Partner Facility at Elettra Sincrotrone Trieste, researchers analyzed the samples structures: they discovered that both samples displayed a cooperative Jahn-Teller-distortion effect (which reduces symmetry and energy in such systems), but also that the sample with the lower vacancies shows a more stable structural change and weaker distortion effect on the Mn sites during the cycling.
Scientists could then explain the better electrochemical performance of the electrode realized with the material with lower vacancy, because the weak Jahn-Teller -distortion effect results in a stable crystal structure during Na+ release and insertion. These findings could lead, in the future, to the development of more stable and performing Na-ion batteries.
ORIGINAL ARTICLE:



