New insights on catalytic nanoparticles synthesis
|Efficient catalysts are able to facilitate the chemical reactions that split water molecules into hydrogen and oxygen and vice versa, i.e. the conversion of hydrogen fuel into electric energy. Therefore, catalysts are essential for green hydrogen-based energy technologies using renewable power coming from solar or wind generators. Importantly, their performance is largely determined by their surface properties.
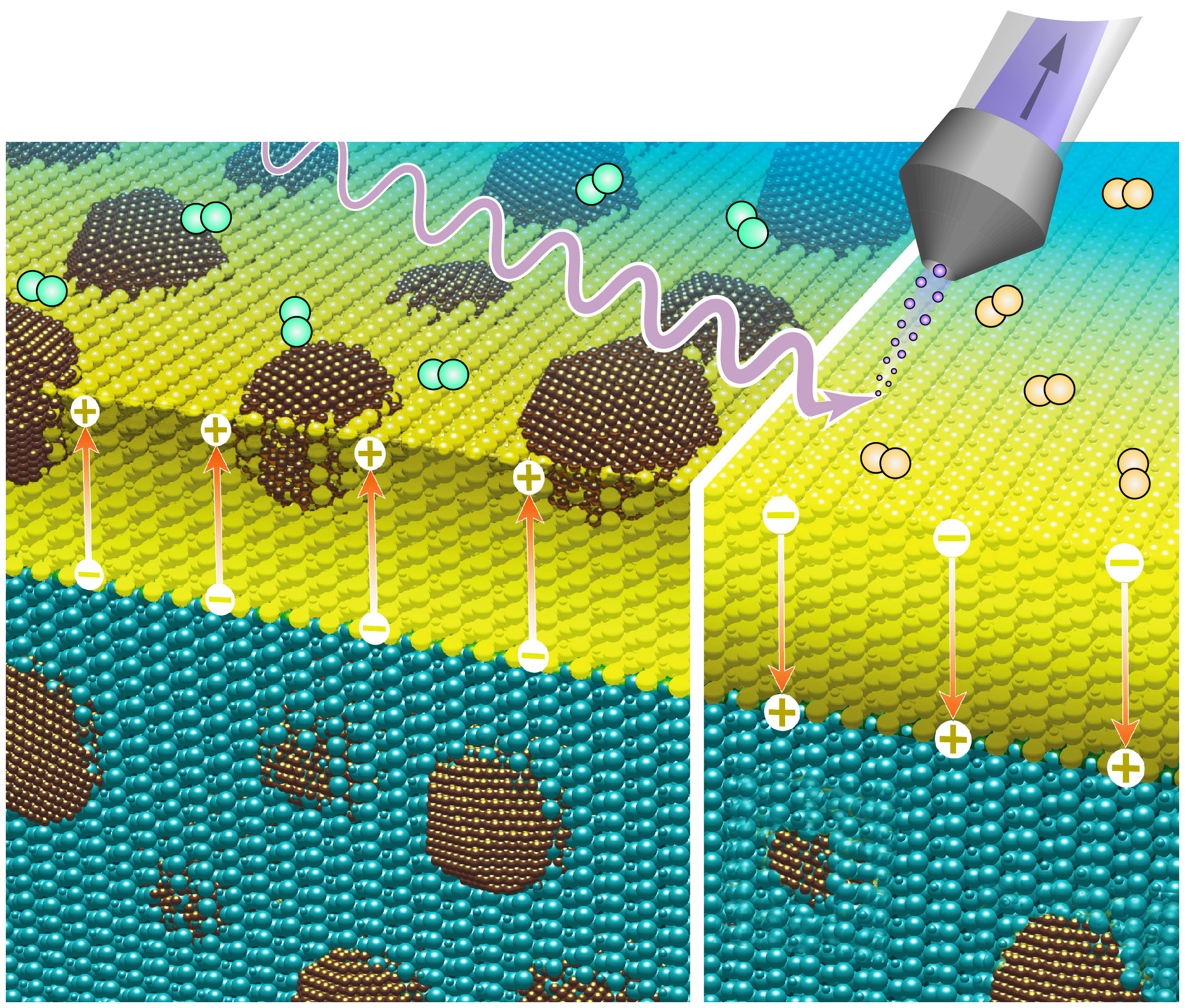
Within this context Dr Moritz Weber, Dr Felix Gunkel, Dr Christian Lenser and colleagues from the Forschungszentrum Jülich (FZJ) described the complex reactions that take place during metal exsolution – a solid-state reaction where metallic nanoparticles segregate from a functional oxide, allowing to obtain a tailored design of catalyst particles – on an atomic level. They explain the fundamental processes that occur during the formation of the nanoparticles and demonstrate that the kinetics of such reactions depends on the electrostatic interaction between the exsolution-active species and the electric field at the surface of the underlying functional oxide. To do so, FZJ researchers – supported by Dr Smid Bretislav, Charles University of Prague – used near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS) available at the Czech CERIC Partner Facility.
These findings represent a significant improvement in the understanding and degree of control of nanoparticles in metal exsolution catalysts, particularly relevant for high-temperature fuel and electrolysis cells, and pave the way for the development of catalysts based on abundant metals, eliminating the need for expensive and hard to come by noble metals.
ORIGINAL ARTICLE:



