Understanding metals interactions in electrolyzers to produce green hydrogen
|Hydrogen produced through water electrolysis is a promising CO2 emissions-free alternative to fossil fuels. Proton Exchange Membrane Water Electrolyzers (PEM-WE) are commonly used to achieve green hydrogen; however, to facilitate their market application, it is crucial to reduce the amount of expensive noble metals, such as iridium (Ir), used by these devices.
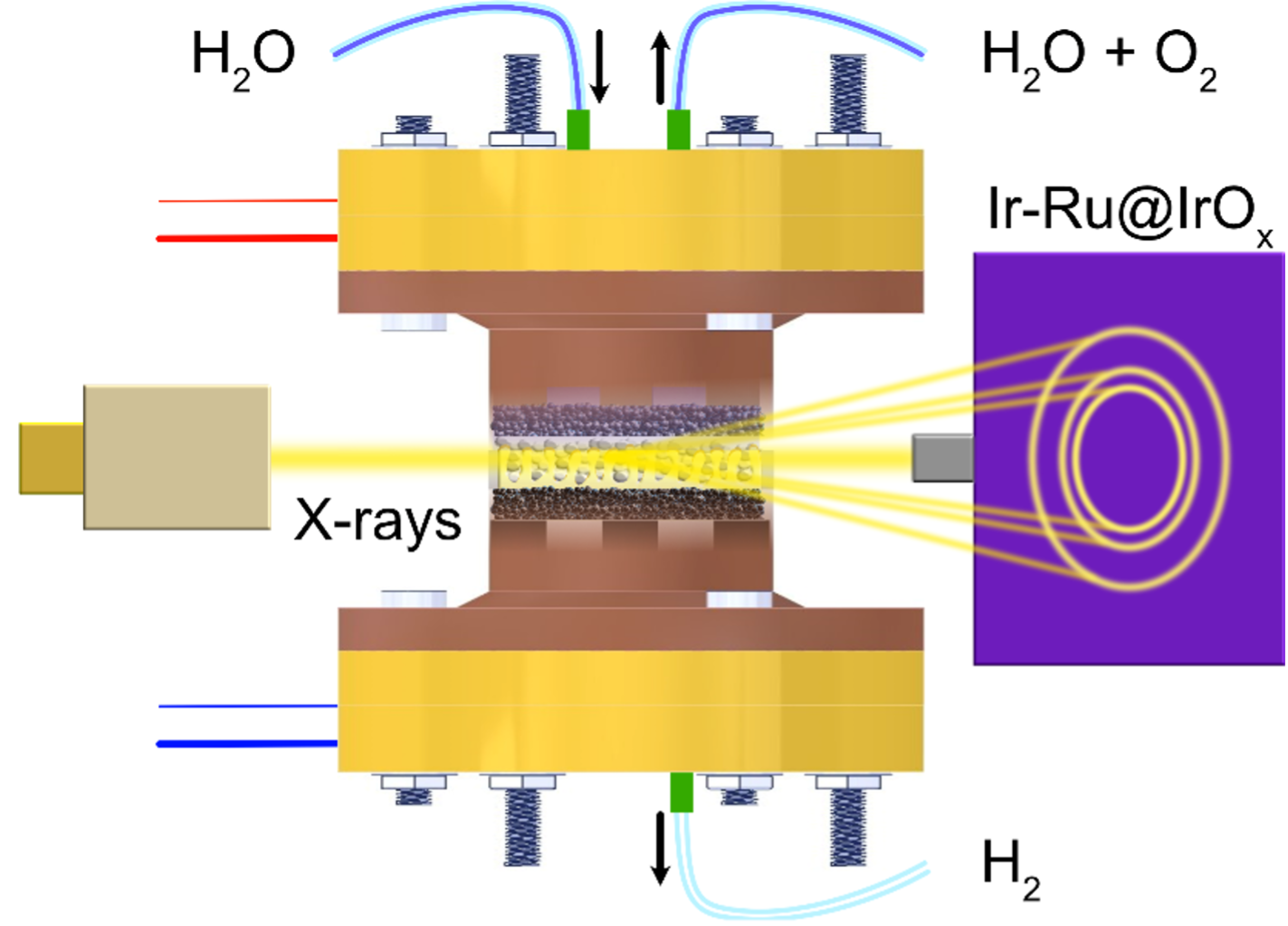
In this context, bimetallic Ir-metal catalysts, such as Ir-Ru or Ir-Ni, proved comparable or higher activities as purely Ir-based ones, yet with much lower Ir loading. A possible explanation of this effect is that the crystallographic structure of catalysts affects their electronic structure and, ultimately, their catalytic activity. To test this hypothesis, Dr Tomáš Hrbek, Dr Peter Kus and colleagues of the Nanomaterials Group at the Charles University in Prague used different, complementary techniques available at the CERIC Czech Partner Facility: the Electron Spectroscopy for Chemical Analysis under Environmental Conditions (EnviroESCA), the High Resolution Field Emmision Scanning electron Microscopy (FESEM), and the Synchrotron Radiation Photoelectron Spectroscopy (SRPES) available at the Materials Science Beamline in Elettra Sincrotrone Trieste. They could then understand that, together, Ru and Ir dynamically form a core-shell structure. The IrOx shell, strained by the Ir-Ru core, maintains a lower oxidation state than pure Ir catalyst, leading to superior catalytic activity and stability.
These findings not only clarify the performance-enhancing mechanisms of Ir-Ru catalysts, but also paves the way for the application of other, more economical materials as effective cores in Ir-metal systems applied to PEM-WEs.
ORIGINAL ARTICLE:



