The role of sulfur into glass formation
|Sulfur, a widespread element is characteristically heterovalent, exhibiting a great range in oxidation state (-2 to +6) and forms chemical bonds with both more electropositive and more electronegative elements. Under reducing conditions, sulfur behaves as an anion, forming bonds directly with metal cations, whereas under oxidizing conditions, it is a complex forming cation, and in some compounds of intermediate oxidation state, it occurs in both anionic and cationic forms. The degree of oxidation of sulfur has a significant effect on the process of melting, forming, clarification, glass structure and properties of the final product, i.e., viscosity, mechanical properties and optical properties (light transmittance and color). The solubility of sulfur in the structure of phosphate glass is higher than that in silicate or borosilicate glass.
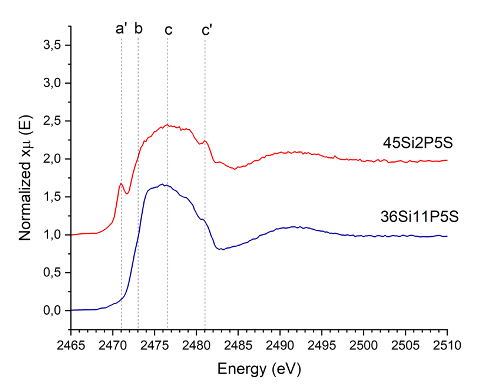
Under reducing conditions, sulfur can incorporate into glass matrices, significantly influencing the polymerization of the glass network and altering their structures, properties and functionality. Dr Justyna Sułowska (AGH University of Krakow, Poland) and colleagues, including Dr Simone Pollastri (Elettra Sincrotrone Trieste, Italy) and Dr Joanna Stępień (AGH University of Krakow, Poland) whose effort has been particularly important for this work, applied a variety of spectroscopic techniques, including X-ray Absorption Fine Structure (XAFS) and X-ray Absorption Spectroscopy (XAS), available at CERIC Italian and Polish Partner facilities, respectively, to identify sulfur’s bonding environment (i.e. whether it is in the form of a sulfate (S⁶⁺) or sulfide (S²⁻)), and understand its behavior in glass matrices. Scientists found that the addition of sulfur increased the ability of glasses with higher amounts of phosphorous oxide to form. Furthermore, XAFS spectra demonstrated that S-bearing glasses contain sulfur in the reduced form as S2-. This finding is shown in the accompanying Figure for glasses containing 45 mol.% SiO2 and 2 mol.% P2O5 (45Si2P5S sample) and 36 mol.% SiO2 and 11 mol.% P2O5 (36Si11P5S sample).
These findings significantly enhance the understanding of sulfur’s structural role in glassy materials, offering insights into how these materials can be optimized for industrial and agricultural uses and paving the way for sustainable applications in material science and beyond. On the basis of spectroscopic studies, it can be also concluded that sulfur ions can scavenge charge-balancing modifier cations from the silicate and phosphate subnetworks.
This work was financed by the National Science Centre, Poland, project number 2018/31/D/ST8/03148 entitled “Chemically active glasses as potential sulphur carriers for the soil environment” (Recipient: Justyna Sułowska)
ORIGINAL ARTICLE:



